TaClpS1, negatively regulates wheat resistance against Puccinia striiformis f. sp. tritici
Qian Yang†, Md Ashraful Islam†, Kunyan Cai, Shuxin Tian, Yan Liu, Zhensheng Kang* and Jun Guo*
Abstract
Background—The degradation of intracellular proteins plays an essential role in plant responses to stressful environments. ClpS1 and E3 ubiquitin ligase function as adaptors for selecting target substrates in caseinolytic peptidase (Clp) proteases pathways and the 26S proteasome system, respectively. Currently, the role of E3 ubiquitin ligase in the plant immune response to pathogens is well defined. However, the role of ClpS1 in the plant immune response to pathogens remains unknown.
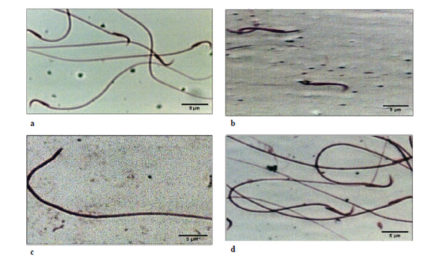
Results—Here, wheat (Triticum aestivum) ClpS1 (TaClpS1) was studied and resulted to encode 161 amino acids, containing a conserved ClpS domain and a chloroplast transit peptide (1–32 aa). TaClpS1 was found to be specifically localized in the chloroplast when expressed transiently in wheat protoplasts. The transcript level of TaClpS1 in wheat was significantly induced during infection by Puccinia striiformis f. sp. tritici (Pst). Knockdown of TaClpS1 via virus-induced gene silencing (VIGS) resulted in an increase in wheat resistance against Pst, accompanied
by an increase in the hypersensitive response (HR), accumulation of reactive oxygen species (ROS) and expression of TaPR1 and TaPR2, and a reduction in the number of haustoria, length of infection hypha and infection area of Pst. Furthermore, heterologous expression of TaClpS1 in Nicotiana benthamiana enhanced the infection by Phytophthora parasitica.
Conclusions—These results suggest that TaClpS1 negatively regulates the resistance of wheat to Pst.
Keywords
Crohn’s disease; Triterpene; TNF-α; NF-κB; FAHF-2